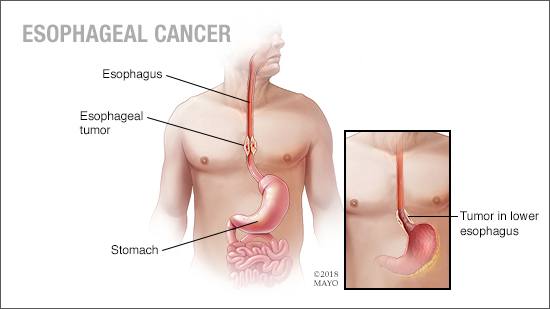
Editor’s note: April is Esophageal Cancer Awareness Month.
Esophageal cancer accounts for only about 1% of new cancer cases in the U.S., far lower than rates in other parts of the world. However, survival rates are low because it is often found at a later stage when symptoms such as difficulty swallowing, worsening heartburn, coughing and hoarseness have begun.
Having precancerous changes in the cells of the esophagus, a condition called Barrett’s esophagus is a risk factor for esophageal cancer. Barrett’s esophagus is caused by gastroesophageal reflux disease (GERD), which occurs when stomach acid repeatedly flows back into the esophagus, irritating the lining of the esophagus.
Risk factors for Barrett’s esophagus include:
- Having a family history of Barrett’s esophagus or esophageal cancer.
- Being male.
- Being white.
- Being older than 50.
- Having chronic heartburn and acid reflux.
- Being a smoker or former smoker.
- Being overweight.
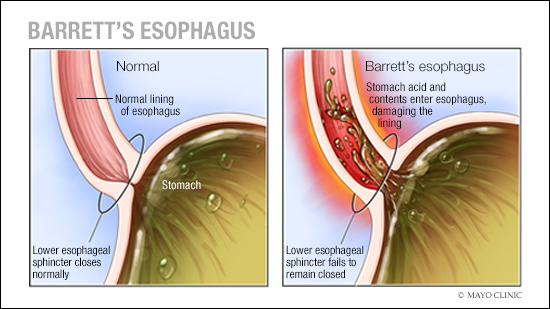
Esophageal cancer specialists recommend screening for Barrett’s esophagus in people who have multiple risk factors. Screening involves a procedure called an endoscopy. During an endoscopy, a healthcare professional passes a lighted tube with a camera at the end (endoscope) down your throat to check for signs of changing esophagus tissue.
Despite these recommendations, screening rates for Barrett’s esophagus are low. Prasad Iyer, M.D., a Mayo Clinic gastroenterologist and researcher, is working to change that.
“We now have access to new, minimally invasive tools for Barrett’s esophagus screening,” he says. “However, there is a critical need to develop more accurate Barrett’s esophagus and esophageal cancer risk assessment tools that can be easily used with electronic health record data.”
Such tools would help healthcare professionals identify people most likely to benefit from screening.
To address this need, Dr. Iyer and a team of researchers developed and tested a tool that uses artificial intelligence (AI) to predict the risk of Barrett’s esophagus and esophageal cancer based on data from a large database of de-identified electronic health records. The results of their study were published in Clinical and Translational Gastroenterology in 2023.
How the study was conducted
Dr. Iyer and his research team used an AI model developed based on de-identified electronic health records of 6 million Mayo Clinic patients to create a risk prediction tool that can determine Barrett’s esophagus and esophageal cancer risk at least a year before diagnosis.
The risk prediction tool can be integrated with an electronic health record and, when appropriate, prompt a healthcare professional to consider screening a patient for Barrett’s esophagus.
Based on clinical, endoscopy, laboratory and pathology notes in the electronic health records, the researchers identified 8,476 people with Barrett’s esophagus, 1,539 people with esophageal cancer and 252,276 people in the control group. They then used these groups to develop predictive models for the risk prediction tool.
The study results
The results of the study demonstrated that the tool’s predictive models had a high level of accuracy:
- The tool predicted Barrett’s esophagus with 76% sensitivity (proportion of samples correctly identified as negative), 76% specificity (proportion of samples correctly identified as positive), and an area under the receiver-operating curve (AUROC) of 0.84. AUROC is a metric used to measure the quality of predictions produced by an AI model. It ranges from 0 to 1, with 1 being the highest quality.
- The tool predicted esophageal cancer with 84% sensitivity, 70% specificity and an AUROC of 0.84.
- The tool also identified known risk factors for Barrett’s esophagus and esophageal cancer, as well as new risk factors to consider, including coronary artery disease, triglyceride levels and electrolyte levels.
“Our work has demonstrated that it’s possible to create a more accurate risk assessment tool for Barrett’s esophagus and esophageal cancer using AI and electronic health record data.
Prasad Iyer, M.D.

“This tool could be integrated into the electronic health record and combined with a minimally invasive (nonendoscopic) screening tool and used by healthcare professionals in primary care,” he says.
Dr. Iyer notes that more research is needed to clarify when healthcare professionals should recommend screening, as well as to clarify other details. “Testing this risk prediction tool in patients and assessing the tool’s performance are important next steps,” he says.
This story originally was published on the Mayo Clinic Comprehensive Cancer blog.
